Smith+Nephew personalizes robotic-assisted surgery for patients and surgeons with launch of CORIOGRAPH™ Pre-Operative Planning and Modeling Services exclusively for the CORI™ Surgical System
Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today announces the launch of its new CORIOGRAPH Pre-Operative Planning and Modeling Services, providing an unparalleled, personalized solution for surgeons and patients across partial and total knee arthroplasty procedures. It is exclusively for use with the CORI Surgical System - the only orthopaedic robotic-assisted system to offer either intraoperative image-free or image-based registration, enabling the surgeon to choose whether or not to perform a pre-operative MRI scan.
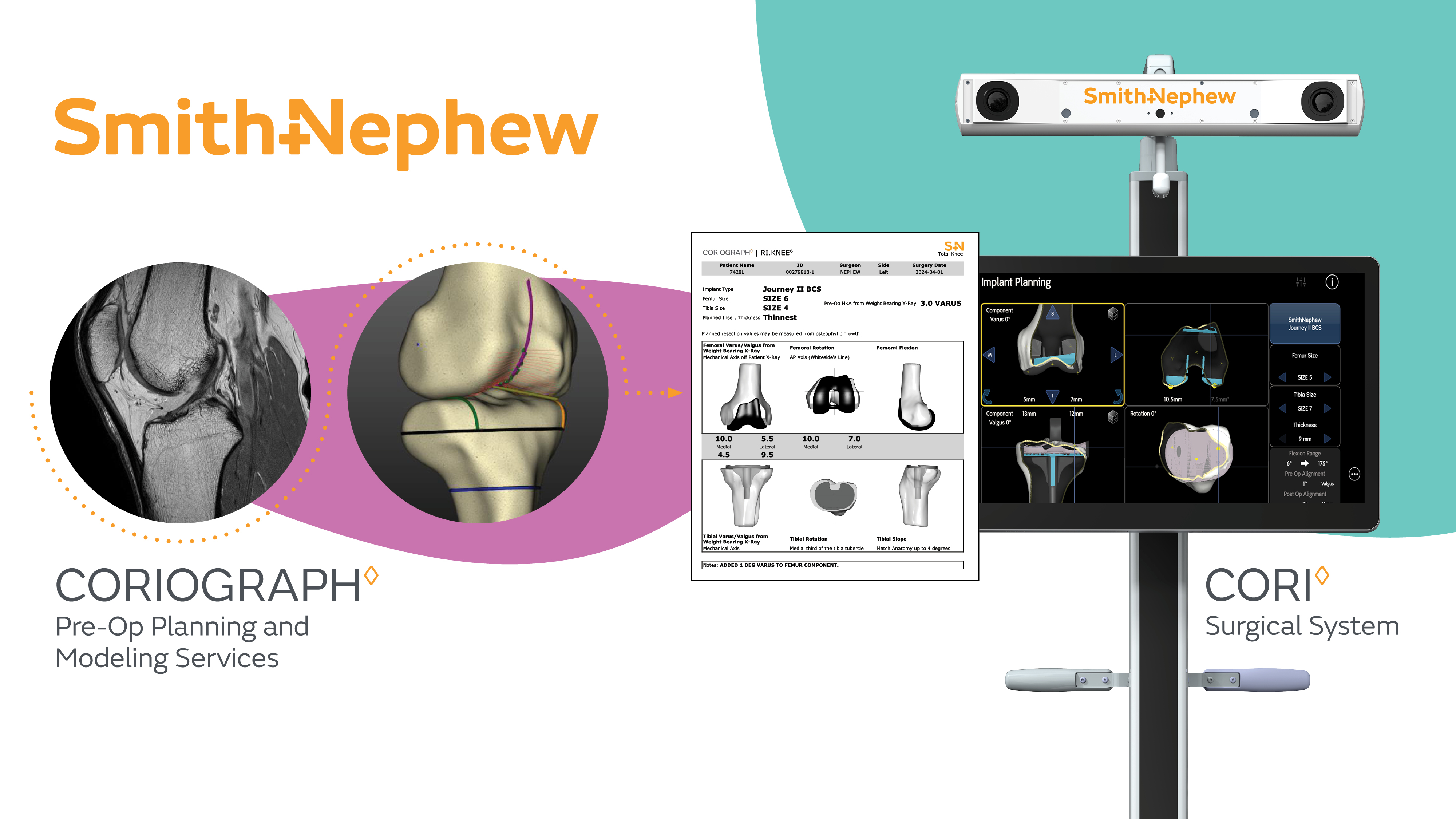
Delivering solutions for differing patient types coupled with planning and execution tools is essential to personalizing surgery. Robotic-assisted surgery can improve accuracy and reproducibility, and may lead to better patient outcomes compared to conventional techniques.1-3
The first procedures using CORIOGRAPH Pre-Operative Planning and Modeling Services and RI.KNEE ROBOTICS 3.0 Software were recently completed by Dr. Steven Haas, Orthopaedic Surgeon and Chief of Knee Service at the Hospital for Special Surgery in New York. He stated, “We are now truly personalizing surgery and advancing efficiencies with RI.KNEE 3.0 and CORIOGRAPH services. Providing the ability to choose the right imaging modality represents the next paradigm for individualized patient care and brings orthopaedic robotics to another level.”
The CORI Surgical System offers proprietary tools and AI-driven software across the full suite of procedure solutions to deliver a robotics platform that is flexible and scalable across joint arthroplasty indications. Built on 15+ years of clinical expertise and over 350,000 image-based surgery plans, CORIOGRAPH Pre-Operative Planning and Modeling Services is where the surgical journey begins. It is designed to optimize procedures and enable intraoperative efficiencies in conjunction with new RI.KNEE ROBOTICS 3.0 Software, which includes:
- Image-agnostic registration functionality, including image-based and image-free offerings
- Optimized knee offering with support for pre-cut tensioning with the CORI Digital Tensioner for partial, total and revision knees
“The introduction of CORIOGRAPH Pre-Operative Planning and Modeling Services signals the next big step in personalized surgery and the evolution of our robotics ecosystem. This combined with the CORI Digital Tensioner will further advance efficiencies for robotic-assisted procedures,” said Mayank Shandil, SVP, Global Marketing – Reconstruction and Robotics at Smith+Nephew.
To learn more about the CORI Surgical System and Smith+Nephew’s digital surgery applications across a range of joint arthroplasty indications, please visit https://www.smith-nephew.com/en/health-care-professionals/products/orthopaedics/cori
- ends –
Enquiries
Media
Dave Snyder +1 (978) 749-1440
Smith+Nephew david.snyder@smith-nephew.com
References
- Matsumoto T, Nakano N, Hayashi S, et al. Prosthetic orientation, limb alignment, and soft tissue balance with bi-cruciate stabilized total knee arthroplasty: a comparison between the handheld robot and conventional techniques. International Orthopaedics 2023;47(6):1473-80. doi: 10.1007/s00264-023-05737-6
- Crizer MP, Haffar A, Battenberg A, et al. Robotic Assistance in Unicompartmental Knee Arthroplasty Results in Superior Early Functional Recovery and Is More Likely to Meet Patient Expectations. Advances in Orthopedics 2021;2021 doi: 10.1155/2021/4770960
- Negrín R, Duboy J, Iñiguez M, et al. Robotic-assisted vs conventional surgery in medial unicompartmental knee arthroplasty: a clinical and radiological study. Knee Surg Relat Res 2021;33(1):5. doi: 10.1186/s43019-021-00087-2 [published Online First: 2021/02/14]
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 18,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global business units of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $5.5 billion in 2023. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on X, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading profit margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: conflicts in Europe and the Middle East, economic and financial conditions in the markets we serve, especially those affecting healthcare providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal and financial compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and disposals, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; relationships with healthcare professionals; reliance on information technology and cybersecurity; disruptions due to natural disasters, weather and climate change related events; changes in customer and other stakeholder sustainability expectations; changes in taxation regulations; effects of foreign exchange volatility; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, which is available on the SEC’s website at www. sec.gov, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain marks registered in US Patent and Trademark Office.

Latest directors dealings
- 16 hours ago IntegraFin Holding
- 16 hours ago Spirent Communications
- 16 hours ago CRH (CDI)
- 17 hours ago Bezant Resources
- 18 hours ago EJF Investments Ltd NPV




